| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Dec 9, 2021~ Largest gene therapy study in hemophilia B achieved primary endpoint of non-inferiority in annualized bleeding rate after stable Factor IX (FIX) expression, assessed at 18 months following a single dose of etranacogene dezaparvovec
Lexington, MA and Amsterdam, the Netherlands, King of Prussia, PA , December 9, 2021 — CSL Behring, a global biotherapeutics leader, and uniQure N.V. (NASDAQ: QURE), a leading gene therapy...
-
Oct 19, 2021• CSL advances novel pipeline spanning six therapeutic areas, four scientific platforms and two businesses (CSL Behring and Seqirus)
MELBOURNE, AU and KING OF PRUSSIA, PA; October 19/18, 2021 – During its annual R&D investment briefing earlier today, CSL Limited (ASX:CSL; USOTC:CSLLY) highlighted progress from its novel...
-
Oct 5, 2021CSL introduced a scholarship program for U.S. employees and their dependents as part of delivering on its promise to diversity, equity and inclusion.
CSL introduced a scholarship program for U.S. employees and their dependents as part of delivering on its promise to diversity, equity and inclusion. 05 Oct 2021 Global biotech leader CSL has...
-
Sep 17, 2021Here's how CSL is working around the world with academia, industry and governments to combat COVID-19
CSL has remained agile to stay ahead of the pandemic challenges and has redirected resources to where it can add the most value to address the pandemic challenges. From the time the coronavirus...
-
Sep 11, 2021Global biotech leader CSL and the Urban League of Philadelphia are working to address some of the most pressing needs in the community, including efforts to strengthen Public Health, Leadership Development, Workforce Diversity and Job Creation and Training.
Global biotech leader CSL and the Urban League of Philadelphia are working to address some of the most pressing needs in the community, including efforts to strengthen Public Health, Leadership...
-
Aug 9, 2021The updated guideline empowers physicians with the confidence to expand the treatment options prescribed for CIDP patients
The updated guideline empowers physicians with the confidence to expand the treatment options prescribed for CIDP patients 09 Aug 2021 KING OF PRUSSIA, Pa. – Aug. 9, 2021 – Global...
-
Aug 3, 2021Approval received to co-package a convenience administration kit along with the product BERINERT.
KING OF PRUSSIA, PA 03 Aug 2021 CSL Behring, a global biotherapeutics leader received U.S. Food and Drug Administration (FDA) approval for its supplemental request (submitted Aug. 30, 2020) for...
-
Jun 8, 2021Hizentra will be covered under the same Medicare benefit category as intravenous immune globulin (IVIg), but with the convenience of self-infusing at home
KING OF PRUSSIA, Pa. 08 Jun 2021 Global biotherapeutics leader CSL Behring today announced that the Centers for Medicare & Medicaid Services (CMS) has approved Hizentra for coverage under Medicare...
-
Jun 2, 2021CSL continues to provide medicines to patients around the world.
Safeguarding our people, patients and donors remains our top priority. As the COVID-19 pandemic evolves, CSL continues to provide medicines to patients around the world. We’re also exploring new...
-
May 16, 2021Findings signal need for novel treatment strategies to provide more protection during this high-risk, 90-day period
KING OF PRUSSIA, Pa. 16 May 2021 Global biotherapeutics leader CSL Behring today announced the results of a new meta-analysis of seven interventional Phase 3 clinical trials that included more...
-
May 13, 2021Dr. Minnie Sarwal of NephroSant has been selected to receive the $25,000 Innovation Award
NEW YORK, NY, USA – May 13, 2021 – Lyfebulb, a patient-empowerment innovation accelerator that bridges the gap between patient communities and the healthcare industry, and CSL Behring, a...
-
May 6, 2021• Etranacogene dezaparvovec (AMT-061) is an investigational gene therapy that may potentially provide people with hemophilia B with years of functional levels of Factor IX, a blood-clotting protein that prevents excessive bleeding.
KING OF PRUSSIA, PA, USA – MAY 6, 2021 – Global biotherapeutics leader CSL Behring today announced the closing of its global Commercialization and License agreement with uniQure (NASDAQ: QURE) for
-
Apr 29, 2021The label update is based on open label extension data from the landmark PATH (Polyneuropathy And Treatment with Hizentra) study
KING OF PRUSSIA, Pa. – April 29, 2021 – Global biotherapeutics leader CSL Behring today announced that the U.S. Food and Drug Administration (FDA) has approved a label update for Hizentra®...
-
Apr 2, 2021Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), did not meet its endpoints to show efficacy in adults hospitalized with COVID-19
Osaka, JAPAN and King of Prussia, Pa., USA 02 Apr 2021 The CoVIg-19 Plasma Alliance today announced that the Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial...
-
Jan 25, 2021• TransplantLyfe is an online community for those living with an organ transplant, their support partners and donors to share experiences, find one-on-one mentorships and help individuals feel safe as they embrace struggles unique to transplantation, regardless of their physical location.
NEW YORK – (January 25, 2021) – Global biotherapeutics leader CSL Behring, and patient empowerment platform, Lyfebulb, announced today the launch of a first-of-its-kind online community...
-
Dec 9, 2020First and only subcutaneous immunoglobulin (SCIg) approved for maintenance therapy in CIDP qualifies for marketing exclusivity
KING OF PRUSSIA, Pa.– December 9, 2019 – Global biotherapeutics leader CSL Behring announced today that Hizentra received orphan-drug exclusivity from the U.S. Food and Drug Administration...
-
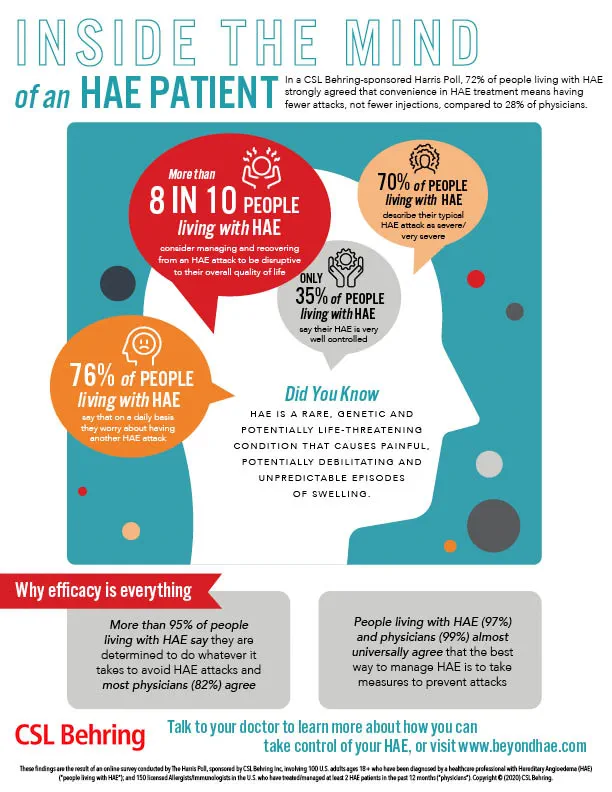
Nov 19, 2020- Reduction in number of attacks is leading factor when evaluating prophylactic therapy
KING OF PRUSSIA, Pa. 19 Nov 2020 CSL Behring, a global biotherapeutics leader, today announced survey results showing that a vast majority of HAE patients (94%) say it’s important their...
-
Nov 16, 2020- 6 Urban League Affiliates across U.S. to participate -- starting with Philadelphia
KING OF PRUSSIA, Pa. and PHILADELPHIA, Pa. 16 Nov 2020 Global biotherapeutics leader CSL Behring today announced a community partnership with six Urban League affiliates across the U.S., starting...
-
Nov 10, 2020Global biotherapeutics leader reinforces commitment to cardiovascular research: Hosts Learning Studio with prominent cardiovascular thought leaders to discuss emerging treatment targets
KING OF PRUSSIA, Pa. 10 Nov 2020 Global biotherapeutics leader CSL Behring today announced that results of two separate analyses will be shared at this year’s American Heart Association (AHA)...
-
Nov 5, 2020The AEGIS-II trial is evaluating the efficacy and safety of CSL112 (apolipoprotein A-1 [human]) during the high-risk 90-day period following a heart attack
KING OF PRUSSIA, Pa. 05 Nov 2020 Global biotherapeutics leader CSL Behring today announced that the study design manuscript for its landmark AEGIS-II (ApoA-I Event reducinG in Ischemic Syndromes...
-
Nov 2, 2020• Both US and European regulators grant special designation
KING OF PRUSSIA, Pa. 02 Nov 2020 Global biotherapeutics leader CSL Behring announced today that its investigational, plasma-derived hemopexin therapy (CSL889) received orphan drug designation from...
-
Oct 29, 2020- Forbes Recognizes Global Biotechnology Leader for Fourth Straight Year
KING OF PRUSSIA, Pa. 29 Oct 2020 Forbes magazine has named global biotechnology leader CSL Limited (parent company of CSL Behring) to its World’s Best Employers 2020 list. It is the fourth year...
-
Oct 20, 2020• Vaccine, hyperimmune and monoclonal antibodies all in clinical stages as potential preventative or treatment options in the fight against COVID-19
MELBOURNE, AU and KING OF PRUSSIA, PA 20 Oct 2020 In its annual R&D briefing to investors today, CSL Limited (ASX:CSL; USOTC:CSLLY) demonstrated how the company is advancing a novel research...
-
Oct 8, 2020• The Alliance’s anti-COVID-19 Hyperimmune Globulin (CoVIg-19) medicine is under evaluation as part of the trial and may become one of the earliest treatments for hospitalized individuals at risk for serious complications of COVID-19
Osaka, JAPAN and King of Prussia, Pa., USA – October 8,2020 – The CoVIg-19 Plasma Alliance, an unprecedented collaboration of leading plasma companies supported by global organizations outside...
-
Sep 28, 2020HAEGARDA is the first and only subcutaneous prophylactic HAE treatment approved for children 6 years of age and older
KING OF PRUSSIA, PA – September 28, 2020 – CSL Behring, a global biotherapeutics leader, announced today that the U.S. Food and Drug Administration (FDA) has approved an expanded indication...