| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
Oct 6, 2025
CSL Behring Canada Inc., a business unit of global biopharma leader CSL, today announced that it has signed a Letter of Intent (LOI) with the pan-Canadian Pharmaceutical Alliance (pCPA) for the...
-
May 15, 2025
CSL Behring’s new facility in Broadmeadows, Victoria, has been named the 2025 Facility of the Year by the International Society for Pharmaceutical Engineering (ISPE), in the Pharma 4.0 category....
-
Apr 18, 2025
ANDEMBRY® received manufacturing and marketing approval in Japan on February 20, 2025, for the prevention of acute attacks in hereditary angioedema (HAE) ANDEMBRY® is the first-in-class...
-
Feb 26, 2025
ANDEMBRY® is the first and only once-monthly treatment targeting factor XIIa to prevent recurrent attacks in HAE patients. The approval marks the fifth regulatory approval of ANDEMBRY,...
-
Feb 13, 2025
- ANDEMBRY®, the first and only once-monthly treatment targeting factor XIIa to prevent attacks in HAE patients, inhibits plasma protein factor XIIa, which initiates the cascade of events leading...
-
Dec 13, 2024
If approved, garadacimab will be the first and only once-monthly treatment inhibiting factor XIIa to prevent attacks in HAE patients – a community CSL has been serving for more than 40 years...
-
Feb 9, 2024
The FDA has been notified of the incident, which occurred at a plasma donation center
-
Jan 16, 2024
Findings show that convenience of treatment and flexibility of administration, such as at-home treatment options, are of high importance to patients KING OF PRUSSIA, Pa., Jan. 16, 2024...
-
Jan 3, 2024
- Hizentra® is the #1 immune globulin prescribed for Primary Immunodeficiency (PI) in the U.S. - Hizentra is the first and only subcutaneous immune globulin (SCIg) treatment approved for Chronic...
-
Jan 2, 2024
- 4- and 5-gram ZEMAIRA® vials may reduce the number of vials necessary for reconstitution, thereby providing convenience and lowering package waste KING OF PRUSSIA, Pa., Jan. 2, 2024...
-
Jun 20, 2023
HEMGENIX®, the first and only FDA approved gene therapy for hemophilia B, has been proven to elevate and sustain factor IX levels for years, significantly reduce the rate of annual bleeds versus...
-
Apr 18, 2023
Hizentra® is the first and only immune globulin (Ig) available in prefilled syringes, offering those living with Primary Immunodeficiency (PI) or Chronic Inflammatory Demyelinating Polyneuropathy...
-
Sep 26, 2022
CSL Behring K.K. (Head Office: Minato-ku, Tokyo; President and Representative Director: Jean-Marc Morange) announces that it has received a manufacturing and marketing approval from the Ministry of Health, Labour and Welfare for “Berinert® S.C. Injection 2000,” a lyophilized human C1-esterase inhibitor concentrate...
-
Sep 19, 2022
CSL applauds the United States District Court’s decision to issue a preliminary injunction preventing the United States Customs and Border Protection (CBP) from continuing to enforce its ban on plasma donations by Mexican nationals who enter the U.S. on a B-1/B-2 visa.
-
Sep 8, 2022
TGA’s designation underscores CSL’s promise to develop and deliver a truly unique portfolio of patient-focused therapies for people with rare and serious medical conditions
-
Aug 17, 2022
KING OF PRUSSIA, PA CSL is on track to file for regulatory approvals next year and will present full data set at an upcoming scientific congress 17 Aug 2022 Global biotechnology leader CSL...
-
Jun 24, 2022CSL continues to provide medicines to patients around the world.
CSL continues to provide medicines to patients around the world. 24 Jun 2022 Safeguarding our people, patients and donors remains our top priority. As the COVID-19 pandemic evolves, CSL continues...
-
Jun 9, 2022Acclaimed Photographer Rankin and CSL Behring Team Up to Launch 'Portraits of Progress', an Exhibition Chronicling the Past, Present and Future of Life with Hemophilia
Rankin's first in-person U.S. exhibit in three years showcases portraits and personal stories highlighting the remarkable progress made in understanding and treating hemophilia and hopes for the future
-
May 24, 2022
-- If approved, etranacogene dezaparvovec would be the first gene therapy option for people living with hemophilia B -- This milestone underscores CSL Behring's promise to develop and deliver a...
-
Apr 28, 2022
The 500 million international units (IUs) will include product specifically manufactured for donation allowing for a longer shelf life and enabling more people around the world to access...
-
Mar 28, 2022
Marketing Authorization Application (MAA) for etranacogene dezaparvovec will be reviewed under accelerated assessment and has the potential to be the first gene therapy for patients living with...
-
Mar 2, 2022With 25,000 global employees, CSL is one of 500 Large Employers recognized across 25 industry sectors
Forbes Magazine has named global biotechnology leader CSL among America's Best Employers for 2022. The annual rankings from Forbes and Statista, the world-leading statistics portal and industry...
-
Feb 4, 2022- Data from the largest gene therapy study in Hemophilia B to date shows that etranacogene dezaparvovec is statistically superior in reducing annualized bleeding rate compared to baseline FIX prophylactic therapy
Global biotherapeutics leader CSL Behring today announced positive long-term results from the Phase 3 HOPE-B clinical trial evaluating etranacogene dezaparvovec (EtranaDez), an investigational...
-
Jan 18, 2022Ad hoc release pursuant to Art. 53 LR, Switzerland
CSL Behring AG, Berne, Switzerland, a wholly-owned subsidiary of global biotechnology leader CSL Limited (ASX: CSL; USOTC: CSLLY), today published the offer prospectus regarding its public tender...
-
Dec 15, 2021
KING OF PRUSSIA, PA, USA – DECEMBER 15, 2021 – Global biotherapeutics leader CSL Behring today announced that the Committee for Medicinal Products for Human Use (CHMP), the chief scientific...
-
Dec 9, 2021~ Largest gene therapy study in hemophilia B achieved primary endpoint of non-inferiority in annualized bleeding rate after stable Factor IX (FIX) expression, assessed at 18 months following a single dose of etranacogene dezaparvovec
Lexington, MA and Amsterdam, the Netherlands, King of Prussia, PA , December 9, 2021 — CSL Behring, a global biotherapeutics leader, and uniQure N.V. (NASDAQ: QURE), a leading gene therapy...
-
Oct 19, 2021• CSL advances novel pipeline spanning six therapeutic areas, four scientific platforms and two businesses (CSL Behring and Seqirus)
MELBOURNE, AU and KING OF PRUSSIA, PA; October 19/18, 2021 – During its annual R&D investment briefing earlier today, CSL Limited (ASX:CSL; USOTC:CSLLY) highlighted progress from its novel...
-
Oct 5, 2021CSL introduced a scholarship program for U.S. employees and their dependents as part of delivering on its promise to diversity, equity and inclusion.
CSL introduced a scholarship program for U.S. employees and their dependents as part of delivering on its promise to diversity, equity and inclusion. 05 Oct 2021 Global biotech leader CSL has...
-
Sep 17, 2021Here's how CSL is working around the world with academia, industry and governments to combat COVID-19
CSL has remained agile to stay ahead of the pandemic challenges and has redirected resources to where it can add the most value to address the pandemic challenges. From the time the coronavirus...
-
Sep 11, 2021Global biotech leader CSL and the Urban League of Philadelphia are working to address some of the most pressing needs in the community, including efforts to strengthen Public Health, Leadership Development, Workforce Diversity and Job Creation and Training.
Global biotech leader CSL and the Urban League of Philadelphia are working to address some of the most pressing needs in the community, including efforts to strengthen Public Health, Leadership...
-
Aug 9, 2021The updated guideline empowers physicians with the confidence to expand the treatment options prescribed for CIDP patients
The updated guideline empowers physicians with the confidence to expand the treatment options prescribed for CIDP patients 09 Aug 2021 KING OF PRUSSIA, Pa. – Aug. 9, 2021 – Global...
-
Aug 3, 2021Approval received to co-package a convenience administration kit along with the product BERINERT.
KING OF PRUSSIA, PA 03 Aug 2021 CSL Behring, a global biotherapeutics leader received U.S. Food and Drug Administration (FDA) approval for its supplemental request (submitted Aug. 30, 2020) for...
-
Jun 8, 2021Hizentra will be covered under the same Medicare benefit category as intravenous immune globulin (IVIg), but with the convenience of self-infusing at home
KING OF PRUSSIA, Pa. 08 Jun 2021 Global biotherapeutics leader CSL Behring today announced that the Centers for Medicare & Medicaid Services (CMS) has approved Hizentra for coverage under Medicare...
-
Jun 2, 2021CSL continues to provide medicines to patients around the world.
Safeguarding our people, patients and donors remains our top priority. As the COVID-19 pandemic evolves, CSL continues to provide medicines to patients around the world. We’re also exploring new...
-
May 16, 2021Findings signal need for novel treatment strategies to provide more protection during this high-risk, 90-day period
KING OF PRUSSIA, Pa. 16 May 2021 Global biotherapeutics leader CSL Behring today announced the results of a new meta-analysis of seven interventional Phase 3 clinical trials that included more...
-
May 13, 2021Dr. Minnie Sarwal of NephroSant has been selected to receive the $25,000 Innovation Award
NEW YORK, NY, USA – May 13, 2021 – Lyfebulb, a patient-empowerment innovation accelerator that bridges the gap between patient communities and the healthcare industry, and CSL Behring, a...
-
May 6, 2021• Etranacogene dezaparvovec (AMT-061) is an investigational gene therapy that may potentially provide people with hemophilia B with years of functional levels of Factor IX, a blood-clotting protein that prevents excessive bleeding.
KING OF PRUSSIA, PA, USA – MAY 6, 2021 – Global biotherapeutics leader CSL Behring today announced the closing of its global Commercialization and License agreement with uniQure (NASDAQ: QURE) for
-
Apr 29, 2021The label update is based on open label extension data from the landmark PATH (Polyneuropathy And Treatment with Hizentra) study
KING OF PRUSSIA, Pa. – April 29, 2021 – Global biotherapeutics leader CSL Behring today announced that the U.S. Food and Drug Administration (FDA) has approved a label update for Hizentra®...
-
Apr 2, 2021Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), did not meet its endpoints to show efficacy in adults hospitalized with COVID-19
Osaka, JAPAN and King of Prussia, Pa., USA 02 Apr 2021 The CoVIg-19 Plasma Alliance today announced that the Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial...
-
Jan 25, 2021• TransplantLyfe is an online community for those living with an organ transplant, their support partners and donors to share experiences, find one-on-one mentorships and help individuals feel safe as they embrace struggles unique to transplantation, regardless of their physical location.
NEW YORK – (January 25, 2021) – Global biotherapeutics leader CSL Behring, and patient empowerment platform, Lyfebulb, announced today the launch of a first-of-its-kind online community...
-
Dec 9, 2020First and only subcutaneous immunoglobulin (SCIg) approved for maintenance therapy in CIDP qualifies for marketing exclusivity
KING OF PRUSSIA, Pa.– December 9, 2019 – Global biotherapeutics leader CSL Behring announced today that Hizentra received orphan-drug exclusivity from the U.S. Food and Drug Administration...
-
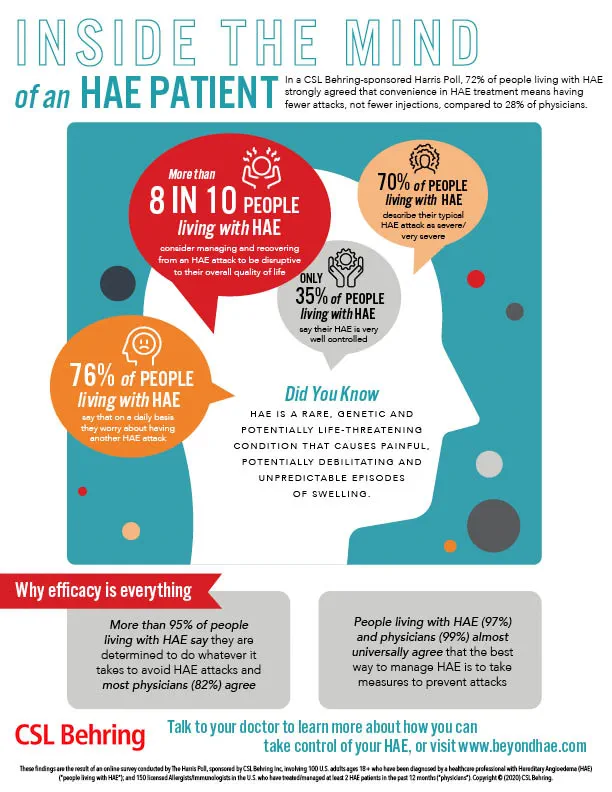
Nov 19, 2020- Reduction in number of attacks is leading factor when evaluating prophylactic therapy
KING OF PRUSSIA, Pa. 19 Nov 2020 CSL Behring, a global biotherapeutics leader, today announced survey results showing that a vast majority of HAE patients (94%) say it’s important their...
-
Nov 16, 2020- 6 Urban League Affiliates across U.S. to participate -- starting with Philadelphia
KING OF PRUSSIA, Pa. and PHILADELPHIA, Pa. 16 Nov 2020 Global biotherapeutics leader CSL Behring today announced a community partnership with six Urban League affiliates across the U.S., starting...
-
Nov 10, 2020Global biotherapeutics leader reinforces commitment to cardiovascular research: Hosts Learning Studio with prominent cardiovascular thought leaders to discuss emerging treatment targets
KING OF PRUSSIA, Pa. 10 Nov 2020 Global biotherapeutics leader CSL Behring today announced that results of two separate analyses will be shared at this year’s American Heart Association (AHA)...
-
Nov 5, 2020The AEGIS-II trial is evaluating the efficacy and safety of CSL112 (apolipoprotein A-1 [human]) during the high-risk 90-day period following a heart attack
KING OF PRUSSIA, Pa. 05 Nov 2020 Global biotherapeutics leader CSL Behring today announced that the study design manuscript for its landmark AEGIS-II (ApoA-I Event reducinG in Ischemic Syndromes...
-
Nov 2, 2020• Both US and European regulators grant special designation
KING OF PRUSSIA, Pa. 02 Nov 2020 Global biotherapeutics leader CSL Behring announced today that its investigational, plasma-derived hemopexin therapy (CSL889) received orphan drug designation from...
-
Oct 29, 2020- Forbes Recognizes Global Biotechnology Leader for Fourth Straight Year
KING OF PRUSSIA, Pa. 29 Oct 2020 Forbes magazine has named global biotechnology leader CSL Limited (parent company of CSL Behring) to its World’s Best Employers 2020 list. It is the fourth year...
-
Oct 20, 2020• Vaccine, hyperimmune and monoclonal antibodies all in clinical stages as potential preventative or treatment options in the fight against COVID-19
MELBOURNE, AU and KING OF PRUSSIA, PA 20 Oct 2020 In its annual R&D briefing to investors today, CSL Limited (ASX:CSL; USOTC:CSLLY) demonstrated how the company is advancing a novel research...
-
Oct 8, 2020• The Alliance’s anti-COVID-19 Hyperimmune Globulin (CoVIg-19) medicine is under evaluation as part of the trial and may become one of the earliest treatments for hospitalized individuals at risk for serious complications of COVID-19
Osaka, JAPAN and King of Prussia, Pa., USA – October 8,2020 – The CoVIg-19 Plasma Alliance, an unprecedented collaboration of leading plasma companies supported by global organizations outside...
-
Sep 28, 2020HAEGARDA is the first and only subcutaneous prophylactic HAE treatment approved for children 6 years of age and older
KING OF PRUSSIA, PA – September 28, 2020 – CSL Behring, a global biotherapeutics leader, announced today that the U.S. Food and Drug Administration (FDA) has approved an expanded indication...
-
Sep 4, 2020- Bronze sponsor CSL Behring also establishes programme for attendees to support Alpha 1 disease awareness
MARBURG, Germany 4 September 2020 - CSL Behring a global biotherapeutics leader announced today that the company will host a symposium focused on the challenges of treating lung disease in the...
-
Jul 30, 2020• On behalf of the CoVIg-19 Plasma Alliance and other plasma companies, Perreault urges people who have recovered from COVID-19 to consider donating plasma. To learn how and where to donate plasma, please visit TheFightIsInUs.org.
Washington, D.C., July 30, 2020 – At today’s White House Roundtable, plasma industry leader and CSL CEO Paul Perreault urged people who have recovered from COVID-19 to consider donating plasma...
-
Jul 9, 2020• Five coagulation researchers named this year’s recipients
KING OF PRUSSIA, Pa. – 9 July 2020 – Global biotherapeutics leader CSL Behring today announced the recipients of its 2020 Professor Heimburger Award for coagulation research just ahead of the...
-
Jul 6, 2020- Phase II placebo-controlled study will assess the safety and efficacy of CSL312 for the treatment of patients with severe respiratory distress due to COVID-19 related pneumonia
KING OF PRUSSIA, Pa. – 6 July 2020 – Global biotherapeutics leader CSL Behring today announced that the first patient has been enrolled in its Phase 2 study to assess the safety and efficacy...
-
Jun 24, 2020• Unique gene therapy has the potential to be one of the first to market treatments to provide potentially long-term benefits with only one dose
KING OF PRUSSIA, Pa., – 24 June 2020 – Global biotherapeutics leader CSL Behring announced today that it has agreed to acquire exclusive global license rights to commercialize an...
-
Jun 19, 2020
PHILADELPHIA--(June 19, 2020) – The University City Science Center announces the election of two new Board members, following the 2020 Annual Meeting of Shareholders held on June 19th: Yi Deng,...
-
Jun 12, 2020Global bleeding disorders community will connect and discuss treatment advances June 14-19, 2020
KING OF PRUSSIA, Pa - June 12 2020 - Global biotherapeutics leader CSL Behring announced today that the company will both attend and sponsor the World Federation of Hemophilia (WFH) first ever...
-
Jun 8, 2020Investigational Monoclonal Antibody Granted U.S. FDA Orphan Drug Designation
HATTERSHEIM, Germany – 8 June 2020 – CSL Behring, a global biotherapeutics leader, today announced results of a Phase 2 clinical trial for garadacimab (previously known as CSL312), an...
-
Jun 8, 2020• Builds on CSL Behring’s promise and commitment to the transplant community
KING OF PRUSSIA, Pa. – 8 June 2020 – Global biotherapeutics leader CSL Behring announced today that it has agreed to acquire Vitaeris Inc., a clinical-stage biotechnology company focused on...
-
Jun 2, 2020Initially, the alliance will develop treatment options for patients with two rare, life-threatening primary immunodeficiency diseases -- Wiskott-Aldrich Syndrome (WAS) and X-linked Agammaglobulinemia (XLA)
SEATTLE and KING of PRUSSIA, Pa. – June 02, 2020 - Seattle Children’s Research Institute, one of the top pediatric research institutions in the world, and global biotechnology leader CSL...
-
May 27, 2020Thermo Fisher Scientific Inc. and CSL Limited have entered into a strategic partnership to help meet the growing demand for biologic therapies while also accelerating CSL’s broader manufacturing objectives.
WALTHAM, Mass. and KING OF PRUSSIA, Penn. 27 May 2020 Thermo Fisher Scientific Inc. (NYSE:TMO), the world leader in serving science, and global biotechnology company, CSL Limited...
-
May 26, 2020“The Fight Is In Us” Campaign Seeks to Mobilize COVID-19 Survivors to Accelerate the Development of Potentially Lifesaving Therapies
Redmond, Wash., USA, and New York 26 May 2020 A coalition of world-leading medical and research institutions, blood centers, life science companies, technology companies, philanthropic...
-
May 7, 2020- Rapidly expanding support for the Alliance is increasing donations of convalescent plasma to begin clinical production, while NIH collaboration confirms clinical trial approach
Osaka, JAPAN, and King of Prussia, PA, USA – 7 May 2020 – The CoVIg-19 Plasma Alliance, an unprecedented plasma industry collaboration recently established to accelerate the development of a...
-
Apr 21, 2020Study examined the safety and tolerability of faster infusion rates and higher infusion volumes, as well as the feasibility of a new manual push administration method
PHILADELPHIA, PA – April 21, 2020 – Global biotherapeutics leader CSL Behring today announced that a clinical study examining faster infusion rates and higher volumes than currently approved...
-
Apr 8, 2020COVID-19 treatment candidate, a high-potency immunotherapy delivering human polyclonal antibodies targeted to SARS-CoV-2, generated from SAB’s novel platform technology, on-track for clinical evaluation as early as summer
King of Prussia, Pa. and Sioux Falls, S.D. 08 Apr 2020 Global biotherapeutics leader, CSL Behring and innovative human antibody development company SAB Biotherapeutics (SAB) announced today their...
-
Apr 6, 2020Partnership brings together world-leading plasma companies to focus on developing and delivering a hyperimmune immunoglobulin in the global fight against COVID-19
Osaka, JAPAN, and King of Prussia, PA, USA 06 Apr 2020 Biotest, BPL, LFB, and Octapharma have joined an alliance formed by CSL Behring (ASX:CSL/USOTC:CSLLY) and Takeda Pharmaceutical Company...
-
Feb 13, 2020Universities awarded $250,000 research grants as part of the CSL Behring-Science Center Research Acceleration Initiative
Universities awarded $250,000 research grants as part of the CSL Behring-Science Center Research Acceleration Initiative 13 Feb 2020 PHILADELPHIA – February 13, 2020 – Researchers at the...
-
Feb 11, 2020CSL Behring advances Ig clinical trial program to address unmet need in treating serious autoimmune disease
KING OF PRUSSIA, Pa.– February 11, 2020 – Global biotherapeutics leader CSL Behring announced today that the U.S. Food and Drug Administration (FDA) has granted Privigen® (Immune Globulin...
-
Jan 21, 2020This year’s rankings based on independent survey of more than 60,000 U.S. employees across industries
KING OF PRUSSIA, Pa., 21 January 2020 – Forbes Magazine today named CSL Behring among its Best Employers for Diversity 2020. The annual rankings, which measure diversity across six different...
-
Jan 10, 2020
Melbourne, Australia 10 Jan 2020 Melbourne researchers have determined the molecular basis for how an important component of the immune system, called gamma-delta T cells, detects infections and...
-
Jan 9, 2020Chief Executive Officer and Managing Director Paul Perreault will present the company's overview on Monday, January 13, at 3:30 p.m. PT, (6:30 p.m. ET), at the Westin St. Francis Hotel.
King of Prussia, Pa. 09 Jan 2020 Global biotechnology giant CSL Limited, parent company of biotherapeutics leader CSL Behring and world-leading influenza provider Seqirus, will present at the 38th...
-
Dec 9, 2019First and only subcutaneous immunoglobulin (SCIg) approved for maintenance therapy in CIDP qualifies for marketing exclusivity
KING OF PRUSSIA, Pa.– December 9, 2019 – Global biotherapeutics leader CSL Behring announced today that Hizentra received orphan-drug exclusivity from the U.S. Food and Drug Administration...
-
Dec 4, 2019Unique portfolio mix of plasma, cell and gene therapy, recombinant proteins and antibody assets highlighted at Research & Development Briefing
Sydney, Australia 04 Dec 2019 CSL Limited (ASX:CSL; USOTC:CSLLY) is steadily advancing its Research & Development (R&D) pipeline and capabilities to deliver a highly differentiated product...
-
Oct 23, 2019Top 100 CEO rankings based on financial performance as well as environmental, social, and governance (ESG) ratings
KING OF PRUSSIA, Pa. – 23 October 2019 – Harvard Business Review (HBR) today named CSL Limited CEO and Managing Director Paul Perreault among the Top 100 Best Performing CEOs in the world for...
-
Sep 27, 2019• Focuses on Patients Through Disease Awareness Support Activities
MADRID, Spain – 27 September 2019 – Global biotherapeutics leader CSL Behring will host a patient-focused symposium about Alpha 1 Antitrypsin Deficiency (AATD) on Monday, 30 September, during the
-
Jul 1, 2019- Novel real world evidence for AFSTYLA® [Antihemophilic Factor (Recombinant), Single Chain] and IDELVION® ® [Coagulation Factor IX (Recombinant), Albumin Fusion Protein] to be highlighted
Melbourne, Australia - 1 July 2019 - Global biotherapeutics leader CSL Behring announced today that the company will support the presentation of new data from its recombinant coagulation factor...
-
Jun 20, 2019Genoa, Italy
- Eight posters including one oral presentation support ongoing research and clinical experience with Hizentra®, the first and only subcutaneous immunoglobulin (Ig) therapy approved to address...
-
May 16, 2019Recipients from the US and Netherlands will be recognized in Marburg, Germany on Friday, 24 May
16 May 2019 Marburg, Germany - 16 May 2019 - Global biotherapeutics leader CSL Behring today announced that the company has named five recipients of the 2019 Professor Heimburger Award for...
-
May 15, 2019Dr. McKenzie will be accountable for leading CSL’s global end-to-end supply chain organization and its accompanying strategy.
15 May 2019 KING OF PRUSSIA, PA – May 15, 2019 - CSL today named Dr. Paul McKenzie, an accomplished global leader with diverse biotechnology experience, as Chief Operating Officer (COO),...
-
Apr 25, 2019New vial options deliver on CSL Behring’s heritage of innovation by providing more alternatives to patients
25 Apr 2019 KING OF PRUSSIA, Pa. – 25 April 2019 – Global biotherapeutics leader CSL Behring today announced that the US Food and Drug Administration (FDA) has approved 4- and 5-gram vial...
-
Apr 9, 2019Leading global biotech company brings innovative therapies for life-threatening medical conditions to UAE
Dubai, UAE - 9 April, 2019 – CSL Behring, a global biotherapeutics leader, has inaugurated its regional headquarters at Dubai Science Park (DSP), a holistic science-focused business community...
-
Mar 26, 2019CSL Behring announced today that Japan’s Ministry of Health, Labour and Welfare has approved two of its immunoglobulin therapies for the treatment of patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Hizentra® and Privigen®
26 Mar 2019 Hizentra® [human normal immunoglobulin, 20%, subcutaneous] is the first and only subcutaneous immunoglobulin approved for the maintenance treatment of CIDP in Japan, based on findings...
-
Feb 4, 2019Reinforces Commitment to Providing Global Bleeding Disorders Community with Access to Care and Efforts to Increase Awareness
04 Feb 2019 MONTREAL and KING OF PRUSSIA, Pa. – 4 February 2019 – The World Federation of Hemophilia (WFH) and global biotherapeutics leader CSL Behring today announced that the company has...
-
Jan 16, 2019Forbes Recognizes the Global Biotechnology Leader in its Top 50 Best Employers for Diversity 2019
16 Jan 2019 KING OF PRUSSIA, Pa. – January 16, 2019 – Forbes magazine has named global biotechnology leader CSL Limited as one of The Best Employers for Diversity in the United States. CSL,...
-
Jan 7, 2019CSL announced that it will present at the 2019 J.P. Morgan Annual Healthcare Conference.
07 Jan 2019 King of Prussia, Pa – Global biotechnology giant CSL Limited, parent company of biotherapeutics leader CSL Behring and world-leading influenza provider Seqirus, today announced that...
-
Dec 6, 2018Perreault leads the fifth largest biotechnology company in the world with more than 22,000 employees providing lifesaving medicines to patients in more than 60 countries.
06 Dec 2018 KING OF PRUSSIA, Pa. – December 6, 2018 – Paul Perreault, CEO and Managing Director of CSL Limited, is one of the Philadelphia Business Journal’s Most Admired CEOs for 2018,...
-
Nov 8, 2018Results highlight need for new pathways and therapeutic strategies to manage costs and maintain quality of care for multi-vessel disease patients
08 Nov 2018 King of Prussia, Pa. – 08 November 2018 – Global biotherapeutics leader CSL Behring in collaboration with Health Analytics, a prominent health economics and outcomes research...
-
Nov 8, 2018Results highlight need for new pathways and therapeutic strategies to manage costs and maintain quality of care for multi-vessel disease patients
08 Nov 2018 King of Prussia, Pa. – 08 November 2018 – Global biotherapeutics leader CSL Behring in collaboration with Health Analytics, a prominent health economics and outcomes research...
-
Oct 22, 2018Partnership to identify and help commercialize potential new medicines at research and academic institutions across the Greater Philadelphia region
22 Oct 2018 PHILADELPHIA, Pa. – October 22, 2018 – The University City Science Center and global biotechnology leader CSL Behring, which has its operational headquarters in King of Prussia,...
-
Oct 22, 2018ESID platinum sponsor to partner with experts examining causes of secondary immunodeficiency
22 Oct 2018 LISBON, Portugal – 22 October 2018 – Global biotherapeutics leader CSL Behring today announced that it will host a panel of cross-specialty experts at its “Current and Emerging...
-
Oct 9, 2018CSL, the parent company of CSL Behring, today announced an AUD$50,000 donation in support of Indonesian earthquake and tsunami relief efforts.
09 Oct 2018 Melbourne, 9 October, 2018 – CSL, the parent company of CSL Behring, today announced an AUD$50,000 donation in support of Indonesian earthquake and tsunami relief efforts. The...
-
Sep 18, 2018Thomson Reuters Includes the Global Biotechnology Leader in its 2018 Top 100 Global Diversity and Inclusion Index
18 Sep 2018 KING OF PRUSSIA, Pa. – September 18, 2018 – Thomson Reuters has ranked global biotechnology leader CSL Limited one of the top 100 companies in its 2018 global Diversity and...
-
Sep 13, 2018• Hosts Patient-Focused Symposium on Alpha 1 Management • Raises Money to Support Disease Awareness
13 Sep 2018 PARIS, France – 13 September 2018 – Global biotherapeutics leader CSL Behring will host a symposium about Alpha 1 Antitrypsin Deficiency (AATD) on Monday, 17 September, during the 28th
-
Aug 23, 2018- The only treatment for hemophilia B FDA approved for up to 14-day dosing is now available in larger, 3500 IU vial size - IDELVION is now offered in 5 convenient vial sizes to fit any dosing regimen: 250, 500, 1000, 2000 and 3500 IU - New vial option delivers on CSL Behring’s promise to enhance current treatments and provide more alternatives to patients
23 Aug 2018 KING OF PRUSSIA, Pa. – 23 August 2018 – Global biotherapeutics leader CSL Behring today announced that IDELVION® [Coagulation Factor IX (Recombinant), Albumin Fusion Protein...
-
Jul 20, 2018• Presentations include new analysis from the PATH trial, the largest clinical trial in CIDP
• CSL Behring is now the only company to offer a portfolio of biologics to address the unique needs of CIDP patients in the US – Hizentra®, the first and only subcutaneous immunoglobulin...
-
Jul 5, 2018- CSL Behring hosts symposium featuring patient perspective on subcutaneous immunoglobulin in Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) treatment
- CSL Behring offers a portfolio of immunoglobulin therapies to address the unique needs of CIDP patients – Hizentra®, the first and only subcutaneous immunoglobulin option for CIDP patients...
-
Jun 22, 2018Named after coagulation therapy pioneer Prof. Norbert Heimburger, the grant has been awarded this year to researchers from France, Netherlands and the United States.
22 Jun 2018 KING OF PRUSSIA, Pa. – June 22, 2018 – An exciting cross-section of coagulation research is being presented today by the recipients of CSL Behring’s 2018 Prof. Heimburger Award...
-
Jun 15, 2018- CSL Behring offers a portfolio of immunoglobulin therapies to address the unique needs of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) patients - CSL Behring will support an oral presentation and four ePresentations focusing on CIDP treatment during the EAN congress
15 Jun 2018 LISBON, Portugal – 15 June 2018 – Global biotherapeutics leader CSL Behring today announced that it will participate in the 4th Congress of the European Academy of Neurology (EAN)...
-
May 31, 2018• IDELVION 14-day dosing option for some patients, combined with the larger vial size, will improve convenience with fewer vials and less infusions • New vial option delivers on CSL Behring’s promise to enhance current treatments and provide more alternatives to patients
31 May 2018 KING OF PRUSSIA, Pa. – 31 May 2018 – Global biotherapeutics leader CSL Behring today announced that the US Food and Drug Administration (FDA) has approved a 3500 IU (international...
-
May 14, 2018HANJ President David Lechner presented the award to Perreault at the association’s 38th Annual Testimonial Dinner Dance
14 May 2018 East Brunswick, N.J. – May 14, 2018 – CEO and Managing Director of CSL Limited Paul Perreault has been named “Humanitarian Man of the Year” by the Hemophilia Association of New...