Recent News Releases
For investor-related news, see our ASX releases here.
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
May 12, 2021
Onshore mRNA manufacturing will build Australian capability for next generation vaccine technologies Melbourne, Australia: CSL welcomes the Government’s plan to further develop onshore mRNA...
-
May 6, 2021
Shareholders agreed to the Board of Directors’ recommendations for all proposed resolutions Election of Dr. Alexandre LeBeaut and Åsa Riisberg as Independent Directors Dividend of CHF 2.00 approved
-
May 6, 2021
Etranacogene dezaparvovec (AMT-061) is an investigational gene therapy that may potentially provide people with hemophilia B with years of functional levels of Factor IX, a blood-clotting protein...
-
May 6, 2021• Etranacogene dezaparvovec (AMT-061) is an investigational gene therapy that may potentially provide people with hemophilia B with years of functional levels of Factor IX, a blood-clotting protein that prevents excessive bleeding.
KING OF PRUSSIA, PA, USA – MAY 6, 2021 – Global biotherapeutics leader CSL Behring today announced the closing of its global Commercialization and License agreement with uniQure (NASDAQ: QURE) for
-
May 1, 2021
Melbourne, Australia: CSL can confirm that production of the AstraZeneca COVID-19 vaccine for Australia has reached more than a million doses a week - these volumes have been produced for a number...
-
Apr 30, 2021
Met primary efficacy endpoint of non-inferiority versus sevelamer carbonate Velphoro® effective in lowering and maintaining serum phosphorus level Safety profile of Velphoro® confirmed...
-
Apr 29, 2021
Topline data expected in the second half of 2021 St. Gallen, Switzerland, and Uniondale, NY, 29 April 2021 – Vifor Pharma and Angion Biomedica Corp. (NASDAQ: ANGN), today announced completion of...
-
Apr 29, 2021
Four Australian Research Programs to be Fast-Tracked in Partnership with CSL Melbourne, Australia: Four Australian medical researcher programs have been awarded a CSL Research Acceleration...
-
Apr 29, 2021The label update is based on open label extension data from the landmark PATH (Polyneuropathy And Treatment with Hizentra) study
KING OF PRUSSIA, Pa. – April 29, 2021 – Global biotherapeutics leader CSL Behring today announced that the U.S. Food and Drug Administration (FDA) has approved a label update for Hizentra®...
-
Apr 28, 2021
CARE-HK in heart failure (HF) is the first global registry of around 5,000 patients with chronic HF who have or are at high risk for hyperkalemia (HK), in Europe and the US CARE-HK in HF is...
-
Apr 8, 2021
CSL remains committed to meeting its contracted arrangements with the Australian Government and AstraZeneca for locally produced AstraZeneca COVID-19 vaccines. We will continue our focused and...
-
Apr 2, 2021
CSL continues to provide medicines to patients around the world. Safeguarding our people, patients and donors remains our top priority. As the COVID-19 pandemic evolves, CSL continues to provide...
-
Apr 2, 2021
Here is how CSL is working around the world with academia, industry and governments to combat the novel coronavirus COVID-19 CSL and AstraZeneca have agreed for CSL to manufacture approximately 50...
-
Apr 2, 2021Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), did not meet its endpoints to show efficacy in adults hospitalized with COVID-19
Osaka, JAPAN and King of Prussia, Pa., USA 02 Apr 2021 The CoVIg-19 Plasma Alliance today announced that the Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial...
-
Mar 30, 2021
Companies have successfully submitted the EU regulatory application for marketing authorization for difelikefalin If approved, difelikefalin injection will be the first therapy available in Europe...
-
Mar 26, 2021
St. Gallen, Switzerland, 26 March 2021 – Vifor Pharma is pleased to announce that the Board of Directors will propose Åsa Riisberg for election to the Board at the Annual General Meeting on 6...
-
Mar 24, 2021
Melbourne, 24 March 2021 – CSL Limited (ASX:CSL) has released 830,000 locally made doses of the AstraZeneca COVID-19 vaccine, ahead of initial scheduling. The release follows the completion of...
-
Mar 8, 2021
FDA has set Prescription Drug User Fee Act (PDUFA) target action date of 23 August 2021 If approved, KORSUVATM injection would be first therapy for treatment of pr uritus in hemodialysis patients...
-
Mar 3, 2021
Vifor Pharma delivers strong full year results 2020 with an EBITDA of 576 million Swiss Francs representing over 29% growth1 Net sales up 3.7% at constant exchange rates (CER), despite significant...
-
Feb 12, 2021
CSL is pleased to confirm that the final stages of manufacturing of the AstraZeneca COVID-19 vaccine for Australia are planned to commence next week, with first doses on track for release towards...
-
Feb 2, 2021
Dr Alexandre LeBeaut to be proposed to Annual General Meeting for appointment to Board Dr Gianni Zampieriand Gilbert Achermann will not stand for re-election St. Gallen, Switzerland, 02 February 2021
-
Jan 31, 2021
24 healthy volunteers to be recruited for study of plasma-derived COVID-19 Immunoglobulin January 31, 2021, Melbourne: CSL Behring Australia, a subsidiary of CSL Limited, today announced that...
-
Jan 25, 2021• TransplantLyfe is an online community for those living with an organ transplant, their support partners and donors to share experiences, find one-on-one mentorships and help individuals feel safe as they embrace struggles unique to transplantation, regardless of their physical location.
NEW YORK – (January 25, 2021) – Global biotherapeutics leader CSL Behring, and patient empowerment platform, Lyfebulb, announced today the launch of a first-of-its-kind online community...
-
Dec 21, 2020
As in ANCA vasculitis, avacopan demonstrated statistically significant improvement in renal function as measured by eGFR compared to placebo over 26 weeks of blinded treatment The change from...
-
Dec 14, 2020
This news release is intended for Health Professional media only. Maidenhead, UK 14 Dec 2020 Seqirus, a global leader in influenza prevention, today presented new real-world evidence (RWE) at the...
-
Dec 14, 2020
Maidenhead, UK 14 Dec 2020 Seqirus, a global leader in influenza prevention and influenza pandemic response, today presented new late-breaking data from a systematic review and meta-analysis at...
-
Dec 11, 2020
11 Dec 2020 The University of Queensland (UQ) and CSL today announce that the Phase 1 trial of the UQ-CSL v451 COVID-19 vaccine has shown that it elicits a robust response towards the virus and...
-
Dec 9, 2020First and only subcutaneous immunoglobulin (SCIg) approved for maintenance therapy in CIDP qualifies for marketing exclusivity
KING OF PRUSSIA, Pa.– December 9, 2019 – Global biotherapeutics leader CSL Behring announced today that Hizentra received orphan-drug exclusivity from the U.S. Food and Drug Administration...
-
Nov 26, 2020
In its sixth year, a growing international coalition underlines significant harm to health caused by iron deficiency Impact on women moves up political agenda at REYKJAVÍK GLOBAL FORUM - WOMEN...
-
Nov 19, 2020
Veltassa® is a well-tolerated1 and effective2 oral calcium potassium binder for the treatment of hyperkalemia supported by 12 month clinical data Veltassa® will benefit from Fresenius Kabi’s...
-
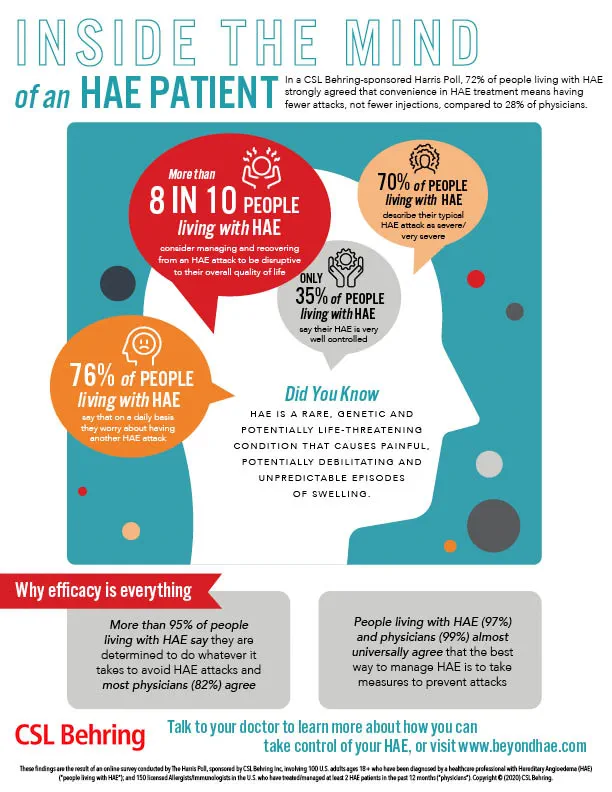
Nov 19, 2020- Reduction in number of attacks is leading factor when evaluating prophylactic therapy
KING OF PRUSSIA, Pa. 19 Nov 2020 CSL Behring, a global biotherapeutics leader, today announced survey results showing that a vast majority of HAE patients (94%) say it’s important their...
-
Nov 16, 2020
16 Nov 2020 Seqirus will build the only cell-based influenza vaccine manufacturing facility in the Southern Hemisphere – producing seasonal and pandemic flu vaccines, Seqirus’ proprietary...
-
Nov 16, 2020- 6 Urban League Affiliates across U.S. to participate -- starting with Philadelphia
KING OF PRUSSIA, Pa. and PHILADELPHIA, Pa. 16 Nov 2020 Global biotherapeutics leader CSL Behring today announced a community partnership with six Urban League affiliates across the U.S., starting...
-
Nov 13, 2020
Ferinject® significantly reduced the incidence of heart failure hospitalizations in patients with iron deficiency after acute heart failure Results of the AFFIRM-AHF trial support the use of...
-
Nov 10, 2020Global biotherapeutics leader reinforces commitment to cardiovascular research: Hosts Learning Studio with prominent cardiovascular thought leaders to discuss emerging treatment targets
KING OF PRUSSIA, Pa. 10 Nov 2020 Global biotherapeutics leader CSL Behring today announced that results of two separate analyses will be shared at this year’s American Heart Association (AHA)...
-
Nov 9, 2020
Vifor Pharma acquires a worldwide license, excluding Greater China, to late-stage product ANG-3777 ANG-3777 is a first in class small-molecule hepatocyte growth factor (HGF) mimetic, addressing a...
-
Nov 8, 2020
CSL Commences Manufacturing of University of Oxford/AstraZeneca Vaccine Candidate in Melbourne. November 8, 2020, Melbourne — CSL Limited (ASX:CSL) CSL today confirmed it will commence...
-
Nov 5, 2020The AEGIS-II trial is evaluating the efficacy and safety of CSL112 (apolipoprotein A-1 [human]) during the high-risk 90-day period following a heart attack
KING OF PRUSSIA, Pa. 05 Nov 2020 Global biotherapeutics leader CSL Behring today announced that the study design manuscript for its landmark AEGIS-II (ApoA-I Event reducinG in Ischemic Syndromes...
-
Nov 3, 2020
Companies have completed the EU regulatory application for marketing approval of avacopan Regulatory submission based on positive data from the pivotal phase-III ADVOCATE trial of avacopan St...
-
Nov 2, 2020• Both US and European regulators grant special designation
KING OF PRUSSIA, Pa. 02 Nov 2020 Global biotherapeutics leader CSL Behring announced today that its investigational, plasma-derived hemopexin therapy (CSL889) received orphan drug designation from...
-
Oct 29, 2020
Forbes Recognizes Global Biotechnology Leader for Fourth Straight Year 29 Oct 2020 Forbes magazine has named global biotechnology leader CSL Limited to its World’s Best Employers 2020 list. It...
-
Oct 29, 2020- Forbes Recognizes Global Biotechnology Leader for Fourth Straight Year
KING OF PRUSSIA, Pa. 29 Oct 2020 Forbes magazine has named global biotechnology leader CSL Limited (parent company of CSL Behring) to its World’s Best Employers 2020 list. It is the fourth year...
-
Oct 20, 2020
Vifor Pharma secures US commercial rights for i.v. Korsuva in non-Fresenius Medical Care dialysis clinics representing approx. 66% of the market, under a profit-sharing arrangement with Cara Cara...
-
Oct 20, 2020• Vaccine, hyperimmune and monoclonal antibodies all in clinical stages as potential preventative or treatment options in the fight against COVID-19
MELBOURNE, AU and KING OF PRUSSIA, PA 20 Oct 2020 In its annual R&D briefing to investors today, CSL Limited (ASX:CSL; USOTC:CSLLY) demonstrated how the company is advancing a novel research...
-
Oct 15, 2020
Two Australian scientists have each been awarded CSL Centenary Fellowships, valued at $1.25 million over five years, to investigate new ways to fight two of the world’s biggest health...
-
Oct 14, 2020
The first annual EU Flu Day was launched today, 14 October 2020. Marking the occasion, Seqirus, a global leader in influenza prevention, announced that it has delivered shipments of influenza...
-
Oct 8, 2020
October 8, 2020, Melbourne — CSL Limited (ASX:CSL; USOTC:CSLLY) today announces that its subsidiary, Seqirus, has signed a final agreement (Agreement) with the Commonwealth of Australia for the...
-
Oct 8, 2020• The Alliance’s anti-COVID-19 Hyperimmune Globulin (CoVIg-19) medicine is under evaluation as part of the trial and may become one of the earliest treatments for hospitalized individuals at risk for serious complications of COVID-19
Osaka, JAPAN and King of Prussia, Pa., USA – October 8,2020 – The CoVIg-19 Plasma Alliance, an unprecedented collaboration of leading plasma companies supported by global organizations outside...
-
Oct 1, 2020
CSL has appointed Ms Joy Linton, a well-respected global leader with extensive strategic and financial experience as Chief Financial Officer (CFO.) Ms Linton will be based at CSL’s Head Office...
-
Sep 28, 2020HAEGARDA is the first and only subcutaneous prophylactic HAE treatment approved for children 6 years of age and older
KING OF PRUSSIA, PA – September 28, 2020 – CSL Behring, a global biotherapeutics leader, announced today that the U.S. Food and Drug Administration (FDA) has approved an expanded indication...